The paper reporting the advance has been published in the journal Advanced Functional Materials. The research was recognized for its excellence and was published as a front cover paper in Advanced Functional Materials. Charging an electric vehicle usually takes about 10 hours or longer, and even with fast-charging methods, it takes at least 30 minutes. That is assuming there is an available spot at a charging station. If we could charge electric vehicle batteries as quickly as refueling gasoline-powered cars, it could help alleviate the shortage of EV charging stations.
The efficiency of lithium-ion batteries, the type used in electric vehicles, is determined by the anode material’s capability to store lithium ions. Recently, Professor Won Bae Kim, from the Department of Chemical Engineering and the Graduate Institute of Ferrous & Energy Materials Technology at Pohang University of Science and Technology (POSTECH, President Moo Hwan Kim), led a research team to develop a new anode material.
By synthesizing “manganese ferrites” anode material into nanosheets with larger surface area more lithium ions can be stored overcoming its theoretical limits. Image Credit: POSTECH. Click the press release link for more images and information.
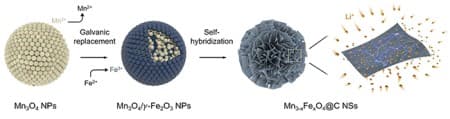
His team, which included Ph.D. candidates Song Kyu Kang and Minho Kim from the Department of Chemical Engineering, synthesized manganese ferrites (Mn3-xFexO4) nanosheets using a novel self-hybridization method involving a straightforward galvanic replacement-derived process. This groundbreaking technique boosts storage capacity approximately 1.5 times above the theoretical limit and enables an electric vehicle to be charged in as little as six minutes.
In this study, the research team devised a new method to synthesize manganese ferrites as anode material known for its superior lithium-ion storage capacity and ferromagnetic properties.
Related: ERCOT Asks Texans To Conserve Energy To Avoid Blackouts
First, a galvanic replacement reaction*1 took place in a solution of manganese oxide mixed with iron, leading to a heterostructure compound with manganese oxide inside and iron oxide outside.
The team further used a hydrothermal method*2 to create nanometer-thick sheets of manganese ferrites with expanded surface areas. This approach harnessed highly spin-polarized electrons, which significantly enhanced the storage capacity for a substantial quantity of lithium ions. This innovation allowed the team to effectively exceed the theoretical capacity of the manganese ferrites anode material by over 50 percent.
Enlarging the surface area of the anode material facilitated the simultaneous movement of a large quantity of lithium ions, thereby improving the battery’s charging speed. Experimental results showed that just six minutes are required to charge and discharge a battery with a capacity equivalent to that used in EVs currently on the market. This study has refined the challenging synthesis process to make a breakthrough in the theoretical capacity of the anode material and significantly accelerate the battery charging process.
Professor Won Bae Kim, the research group’s leader, stated, “We have offered a new understanding on how to overcome the electrochemical limitations of conventional anode materials and increase battery capacity by applying the rational design with surface alteration using electron spin.” He expressed optimism that this development could lead to increased battery durability and reduced recharging time for electric vehicles.
The research was conducted with the support of the Mid-Career Researcher Program and the Advanced Research Center Program of National Research Foundation of Korea and the Ministry of Science and ICT, and the Ministry of Trade, Industry and Energy Program to Upgrade the Performance of Next Generation Rechargeable Lithium-ion Batteries and Develop New Manufacturing Technologies.
- Galvanic replacement reaction
An electrochemical reaction when a metal meets a metal ion with a higher reduction potential. - Hydrothermal method
A method of synthesizing nanoparticles and microparticles by reacting an aqueous solution containing metal ions at high temperature and pressures.
***
This might be a major breakthrough for the field of lithium ion battery chemistry. Should this technology get to commercial scale there will be a new standard for competing technologies to face.
ADVERTISEMENT
A 50 percent increase in capacity would be a major change to even small systems like cell phones. And just staying at the same capacity would save on the total lithium needed.
And a six minute charge? Unheard of until now. Lets hope this technology will scale up easily!
By Brian Westenhaus for Oilprice.com
More Top Reads From Oilprice.com:
- ‘Nuclear Diesel’ Could Become A Gamechanger In Energy Markets
- Saudi Arabia’s Crude Oil Exports Dropped To A Five-Month Low In April
- WTI Drops As Demand Fears Take Over Markets


















